
The resulting number is the number of neutrons in the atom.Vanadium is a chemical element with symbol V and atomic number 23. To do this, round up the atomic weight to the nearest whole number to find the mass number, then subtract the number of protons. You can use this number to figure out things like the number of neutrons in an atom of the element. This number represents the combined weight of all the particles in the atom’s nucleus, including protons and neutrons. Another number that appears in each square is the atomic weight, which is usually written as a whole number with a decimal after it. Unless the element is ionized, the number of protons will be equal to the number of electrons. In addition to the element symbol, you’ll also see the atomic number at the top of the square, which represents the number of protons in an atom of the element. For instance, the symbol for lead is Pb, from the Latin word for lead, plumbum. While some of these are pretty obvious, like O for oxygen and C for carbon, others don’t necessarily match the common name of the element. For example, every element has a 1 to 3-letter symbol that represents the name of the element. When you look at individual elements on the periodic table, there are several pieces of information that you can find on each square. One group of elements that have nearly indistinguishable chemical properties, called the rare earth elements, is separated out into its own separate space at the bottom of the periodic table. This is because the elements don’t always fit neatly into groups that share the same physical and chemical characteristics. Each of these 7 rows is called a “period.” Finally, you might notice that there are gaps in the periodic table, particularly in the top 3 rows. The orbitals are the regions around the atom’s nucleus where electrons are most likely to appear.
When you read the period table in rows going across from left to right, you will find that the elements in each row share the same number of atomic orbitals. Additionally, related elements are usually color-coded to indicate whether they are metals, semi-metals, or non-metals. Each column in the periodic table, going from top to bottom, represents one of these groups.
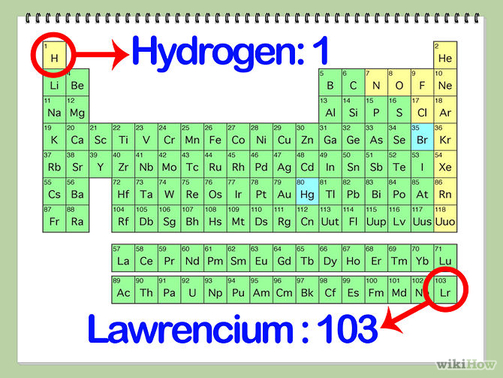
The elements are also organized into groups, or families, that share similar physical and chemical properties. At the other extreme, an atom of the synthetic element oganesson has 118 protons and an atomic number of 118. For instance, the first element in the periodic table, hydrogen, has an atomic number of 1, because a hydrogen atom has only 1 proton. Each element has its own atomic number, which represents the number of protons in one atom of the element. As you move across the table, the number of protons and the atomic mass of each element increases. When you’re reading the periodic table, move across the table from top left to bottom right. But it’s not too difficult to understand once you learn the basics of how it works. The periodic table can seem a little daunting at first.


 0 kommentar(er)
0 kommentar(er)
